Abstract
Background: Current treatment options for R/R AML are highly inadequate. CD33 is expressed in >99% of AML cases. BiTE®s have been effective in R/R Acute Lymphoblastic Leukemia. AMG 330 is a BiTE® that binds CD33 and CD3 on T cells, facilitating T-cell destruction of CD33+ cells. The objectives of this ongoing study are to evaluate the safety, pharmacokinetics, and pharmacodynamics of AMG 330 in R/R AML and to estimate the maximum tolerated dose.
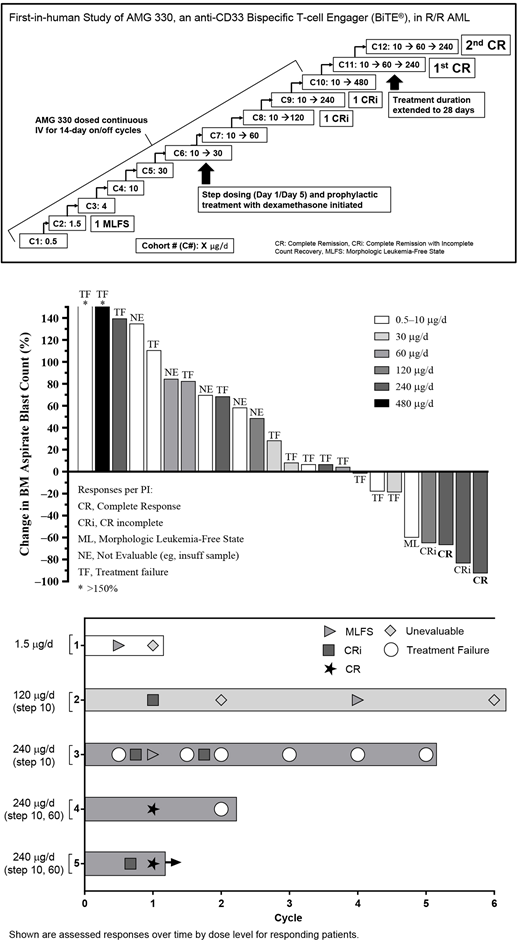
Methods: This was a phase 1 dose escalation study evaluating AMG 330 as a continuous IV infusion in patients with R/R AML, with single-patient cohorts for the first 3 doses and subsequently 3-6 patients per cohort (NCT#02520427). Response was per revised IWG criteria with the addition of complete response (CR) with partial hematologic recovery. After completing the first cycle without dose-limiting toxicity (DLT), up to 5 additional cycles could be given for benefit. After the 30 μg/day (d) cohort, risk mitigation measures for cytokine release syndrome (CRS) were put in place, including step-dosing and pretreatment with a single dose of corticosteroids. The modified treatment regimen consisted of an initial run-in dose of 10 μg/d × 4d followed by the target dose. A 2-step regimen was then tested, ie 10 μg/d, 60 μg/d, and then the target dose, for a treatment duration of 14d or 28d, followed by 1-4 weeks off treatment.
Results: As of June 14, 2018, 35 patients had enrolled in 12 dose cohorts with a target dose range of 0.5-480 μg/d in this ongoing study. Over half (20/35, 57%) of patients were male and the median age was 58 (range: 18-80) years; 14/35 (40%) have previously received a stem cell transplant. Median AML disease duration at baseline was 1.3 (range: 0.3-9.6) years, median proportion of blasts at baseline was 37% (range: 3%-95%), and the median # of prior treatments was 4 (range: 1-15). Median baseline ANC was 0.2 (range: 0-8.6) × 109/L.
Patients received a median of 1 (range: 1-6) cycle with AMG 330; 31/35 (89%) patients discontinued treatment for disease progression (n=24), adverse events (AEs; n=5, 2 treatment-related), and patient request (n=2). One patient completed the maximum of 6 cycles allowed and 3 patients are still receiving study drug. Serious AEs (SAEs) were seen in 23/35 (66%) patients (treatment-related in 15 patients); SAEs seen in >1 patient included CRS (n=11), febrile neutropenia (n=6), pneumonia (n=4), leukopenia (n=3), thrombocytopenia (n=2), and subdural hematoma (n=2); 1 patient died on study due to AML progression (not treatment-related). One patient each in the 10 μg/d and 30 μg/d cohorts (no lead-in) experienced severe CRS; CRS signs and symptoms resolved in 1d with corticosteroids, vasopressors, and IV fluids, and interruption of AMG 330. There were DLTs of grade 2 CRS and grade 4 ventricular fibrillation with a target dose of 480 μg/d; the target dose was then decreased to 240 μg/d.
Two patients had a CR at a target dose of 240 μg/d (lead-in of 10 μg/d→60 μg/d); 1 patient each at target doses of 120 μg/d and 240 μg/d had a CRi and 1 patient who received 1.5 μg/d had a morphologic leukemia-free state (MLFS, <5% blasts, no hematologic recovery). One patient with a CR had bone marrow blasts decrease from ~5%-10% (estimated due to patchy disease) to 2.5% by d29 by flow cytometry, with no morphologic evidence of residual AML and normo- to hypercellular marrow and recovery of peripheral blood counts. The second CR patient had blasts decrease from 40% to 3% with recovery of peripheral blood counts by d42 after receiving one cycle of AMG 330. Correlative data will be presented.
Conclusions: Preliminary data of AMG 330 dosed up to 480 μg/d provide encouraging early evidence of tolerability and anti-leukemic activity in heavily pre-treated patients with R/R AML. Expected CRS was mitigated through step-up dosing, corticosteroid pretreatment, IV fluids, tocilizumab, and drug interruption if needed; most patients had short periods of CRS which responded well to treatment. A 2-step approach will be used in the future to quickly achieve the target dose and optimize clinical response. Regarding pharmacodynamics, to date, 2 CRs and 2 CRis have been observed at target doses of 120 and 240 μg/d. As nearly all patients were substantially cytopenic at baseline, it is challenging to evaluate the impact of AMG 330 on cytopenias. Of note, both CR patients had a complete recovery of blood counts after one cycle of treatment. These promising data validate the use of the BiTE® platform to target CD33.
Ravandi:Sunesis: Honoraria; Bristol-Myers Squibb: Research Funding; Abbvie: Research Funding; Bristol-Myers Squibb: Research Funding; Xencor: Research Funding; Jazz: Honoraria; Orsenix: Honoraria; Xencor: Research Funding; Jazz: Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Seattle Genetics: Research Funding; Macrogenix: Honoraria, Research Funding; Abbvie: Research Funding; Orsenix: Honoraria; Seattle Genetics: Research Funding; Macrogenix: Honoraria, Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Astellas Pharmaceuticals: Consultancy, Honoraria; Sunesis: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau. Stein:Celgene: Speakers Bureau; Amgen: Speakers Bureau. Walter:Amphivena Therapeutics: Consultancy, Equity Ownership, Other: Clinical trial support, Research Funding; Aptevo Therapeutic: Consultancy, Other: Clinical trial support, Research Funding; Covagen AG: Consultancy, Other: Clinical trial support, Research Funding; Seattle Genetics, Inc.: Consultancy, Other: Clinical trial support, Research Funding; Boehringer Ingelheim Pharma GmbH & Co. KG: Consultancy; Pfizer: Consultancy; Amgen Inc.: Other: Clinical trial support, Research Funding; Actinium Pharmaceuticals, Inc.: Other: Clinical trial support, Research Funding. Paschka:Amgen: Other: Travel support; Jazz: Speakers Bureau; Bristol-Meyers Squibb: Other: Travel support, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees; Janssen: Other: Travel support; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Otsuka: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Astellas: Membership on an entity's Board of Directors or advisory committees, Travel support; Astex: Membership on an entity's Board of Directors or advisory committees; Sunesis: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Travel support. Ossenkoppele:Celgene: Honoraria, Research Funding; Roche: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Karyopharm: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Genmab: Research Funding; Johnson & Johnson: Consultancy, Honoraria, Research Funding. Yang:Amgen Inc.: Employment, Equity Ownership. Mehta:Amgen Inc.: Employment, Equity Ownership. Subklewe:Roche: Consultancy, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Celgene: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal